About Us
Our history Laboratoires Reina is a Spanish manufacturer specialising in the development, production and distribution of injectable hyaluronic acid-based medical devices for Aesthetic and Anti-ageing Medicine. Since its opening, Laboratoires Reina has become a major player on the Medical Aesthetics market in Europe. Reina’s product portfolio: Reinafill ®️ dermal fillers.
Research & Development
Laboratoires Reina’s injectable products are designed and developed in the Reina Research & Development Laboratory in Spain, a site that has been entirely dedicated to innovation in the Anti-Ageing and Aesthetics fields. Laboratoires Reina is always seeking to develop innovative, safe and high-quality injectable products that meet patient and doctor expectations and needs, while always remaining compliant with the European and International medical device regulations. The R&D, Production, Quality & Regulatory Affairs teams collaborate to achieve a common goal: Providing high-end, well tolerated and safe injectable products for patients.
Reinafill SAFETY AND EFFECTIVENESS
Reinafill ®️showed its effectiveness and safety, proved by a series of comparative clinical studies as it showed a very low risk of infection, extrusion, migration or granulomatous formation.
Clinical studies
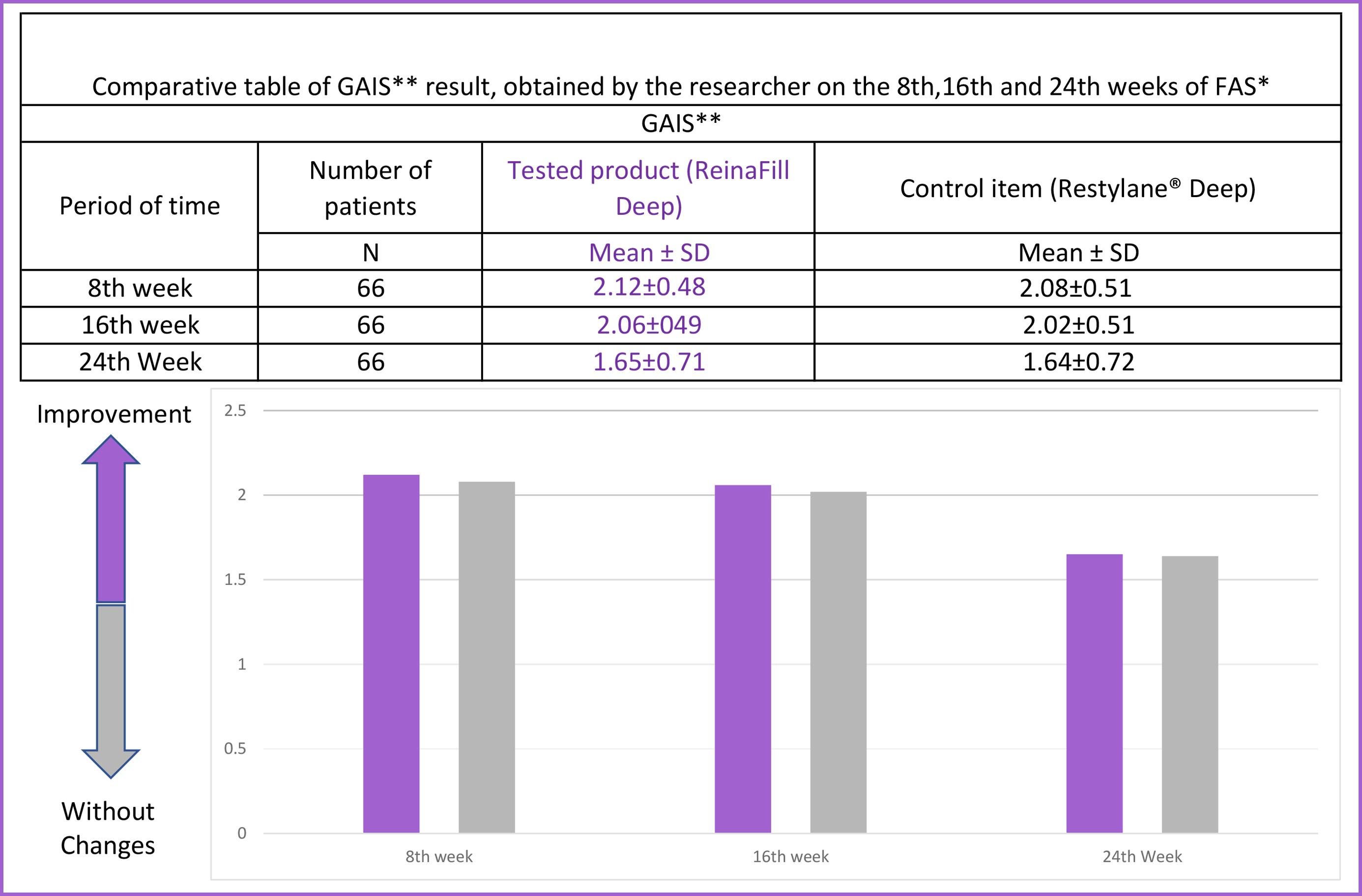
Clinical studies of the medical product Reinafill ®️ Deep have proven their effectiveness and safety for nasolabial folds and lips correction. Participants of the study 66 participants scored 3 or 4 points by GAIS Scale . Target: eliminate nasolabial folds. Methodology Arbitrary sampling; diversified blind study. Control of efficiency and safety of comparing with Restylane®️ (Q Med, Sweden). Conclusion Clinical studies have confirmed that Reinafill is a safe and efficient medical product for nasolabial folds correction,lip filling and face contouring. * FAS = a set of data for a full analysis; * GAIS * Global Aesthetic Improvement Scale Reinafill volume on microscope
Observational Registry
Observational Registry Study to Evaluate Effectiveness and Safety of Reinafill A Prospective, Observational Registry Study to Evaluate Effectiveness and Safety of reinafill Hyaluronic Acid-Based Dermal Fillers in Routine Practice: Interim Analysis Results with One Year of Subject Follow-Up Background: Monitoring the effectiveness, safety and emerging uses of hyaluronic acid (HA) fillers in their wide range of indications requires a holistic approach. Purpose: To propose an observational study design aiming to gather real-world evidence (RWE) and continuously evaluate the performance and safety of marketed devices in routine practice. Materials and Methods: A prospective, observational registry was initiated at six European sites. Investigators enrolled any subject receiving at least one injection with a target study device (Reinafill classic and/or Reinafill deep) They followed their routine practice regarding injection technique, volume, and subject follow-up. Effectiveness was evaluated at 3 months using the global aesthetic improvement scale (GAIS). Safety was assessed based on common treatment reactions (CTR) and adverse events (AE). Results: High quantity of RWE was collected following the initiation of this registry. In the first 158 subjects enrolled, 1220 injections were performed in more than 25 indications, including 679 with the target devices and 271 with devices of the same filler line. The primary objective was achieved, with 93.9% of treatments providing improvement at Month 3 according to the PI and subject. Post-injection CTR were mild to moderate and short-lived, and there was no clinically significant AE. More than 76% of treatments still provided some visible effect at month 12. Conclusion: Based on RWE, Reinafill classic and Reinafill deep are effective and safe in their respective indications mostly distributed in the midface, perioral region, and lower face. Observational registries are a valuable asset in the context of post-market clinical follow-up.
